Proudly Supporting
Top Healthcare Systems
Redefining skin closure with silk fibroin.
Buy SYLKE®
Years On The Market
Sizes Now Available
Peer-Reviewed Studies Published
Happy SYLKE® Patients
The Final Step, The First Impression
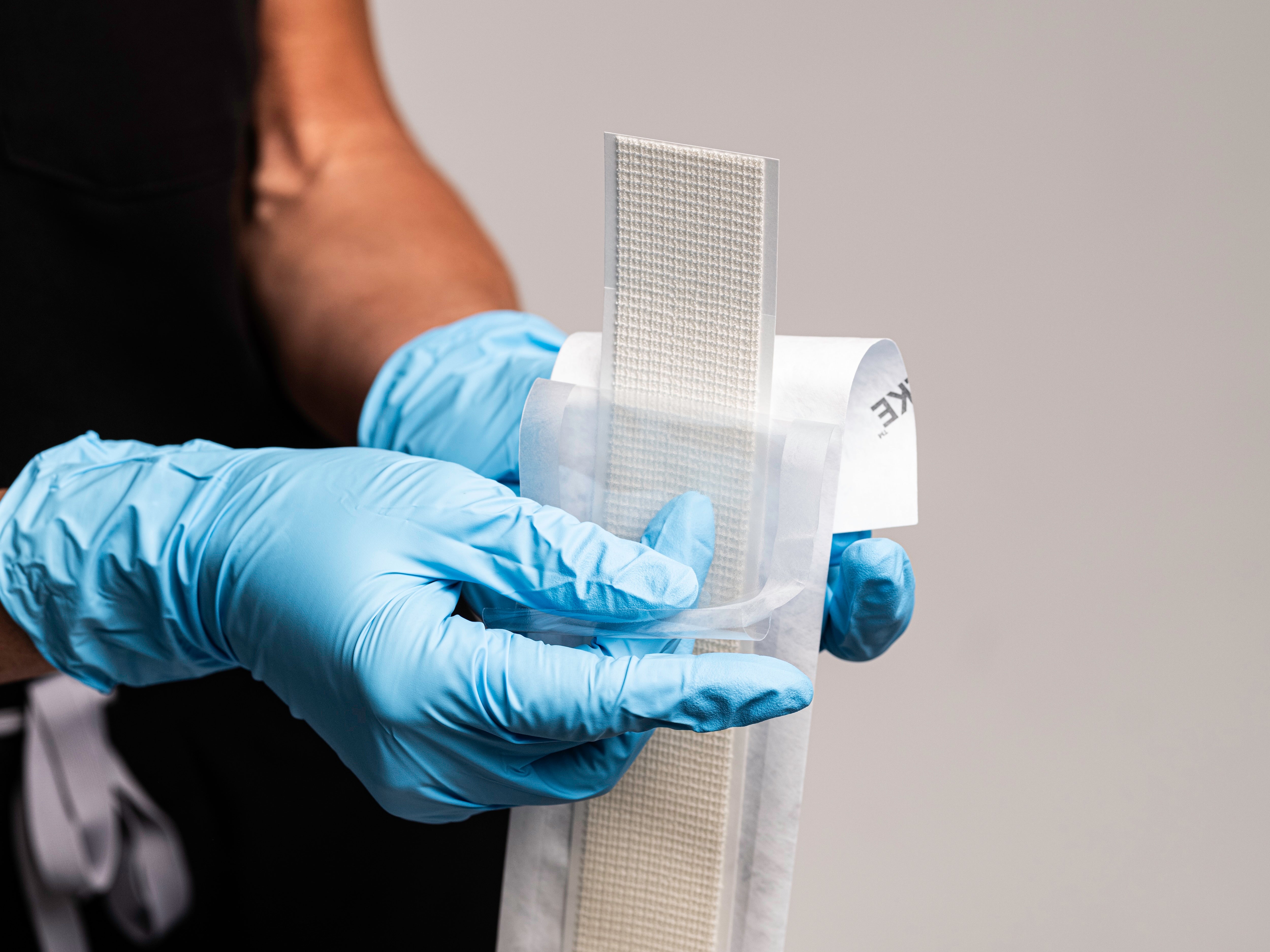
Application & Removal
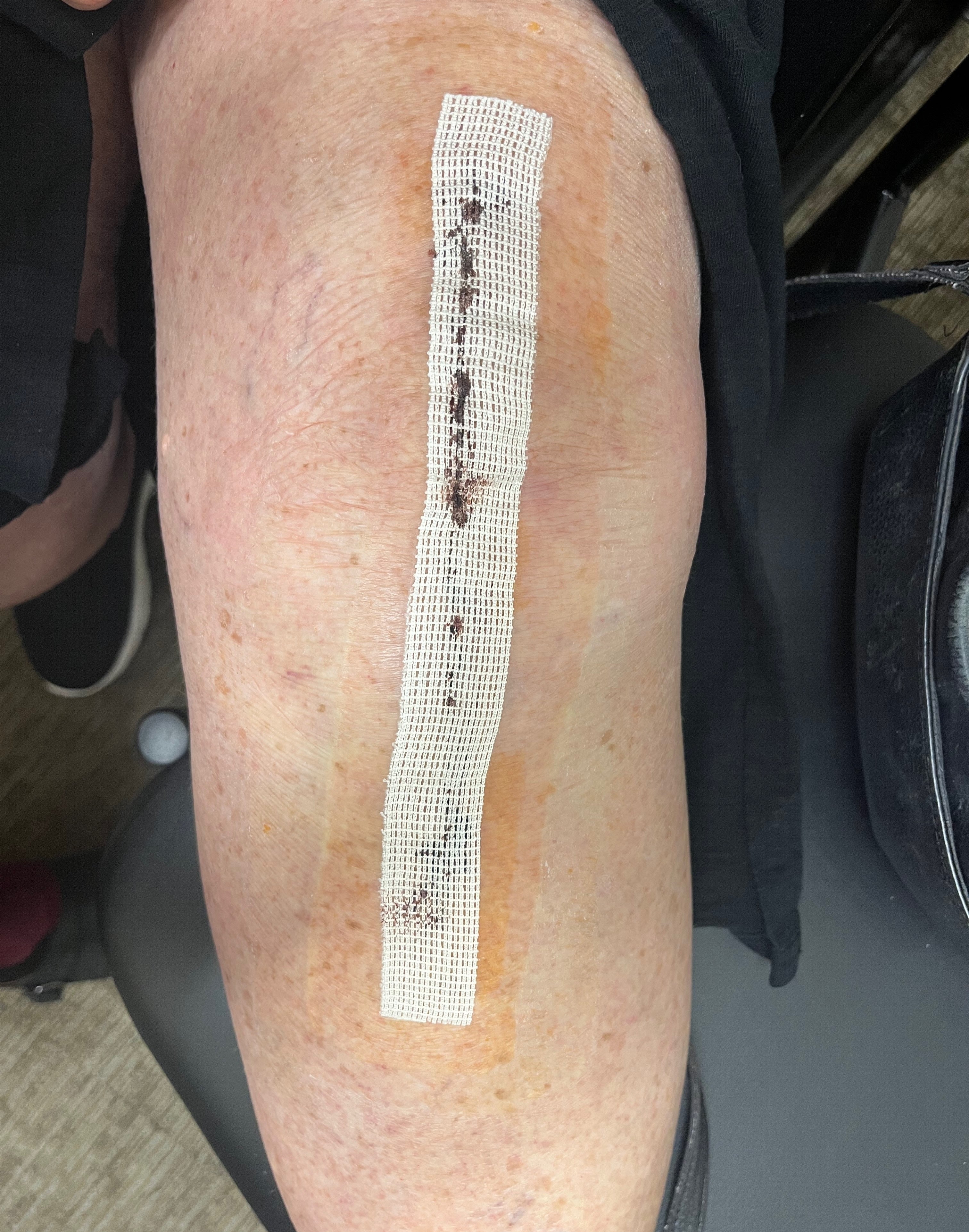
SYLKE®
Application
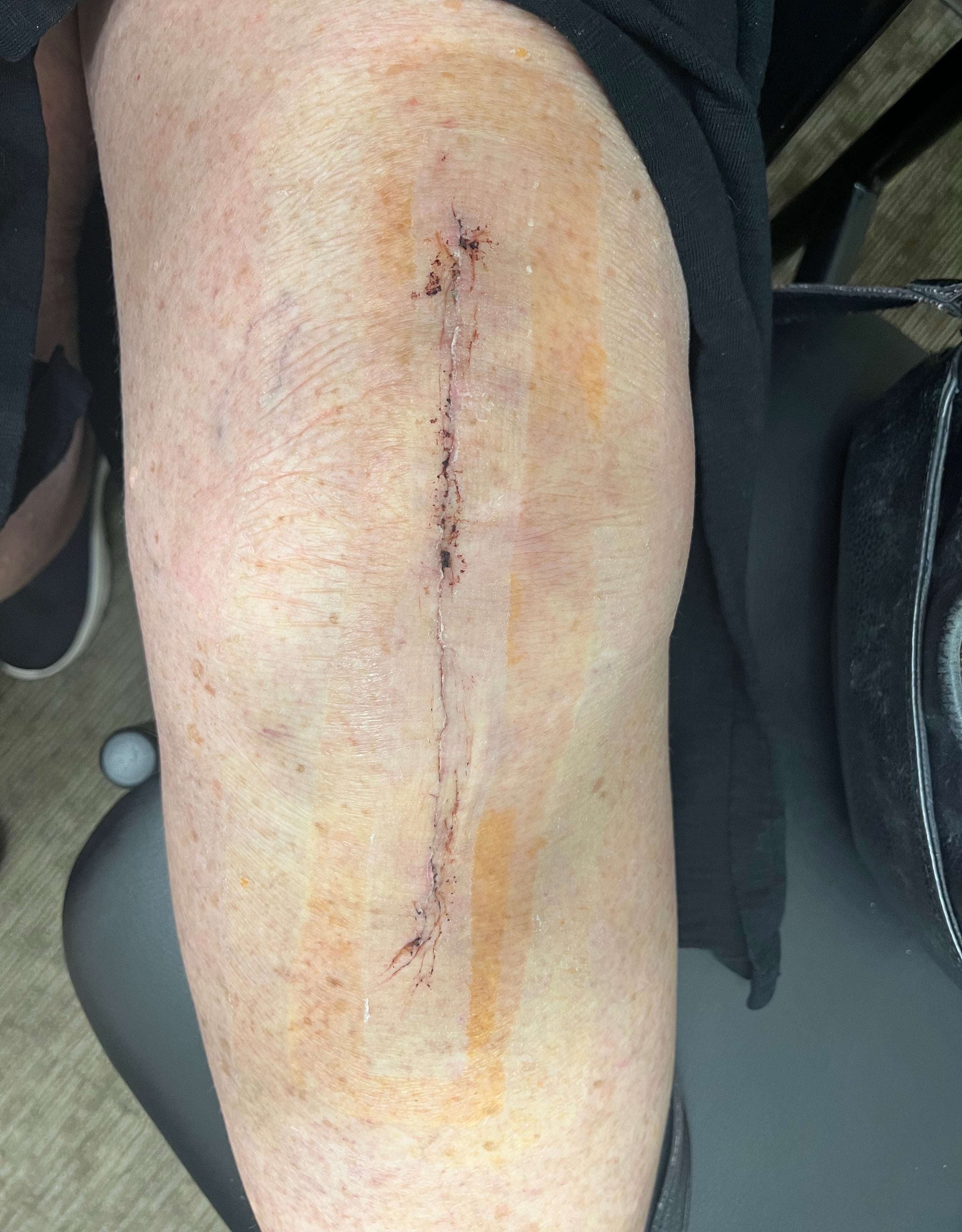
SYLKE®
Removal
Why SYLKE®?
Biocompatible
The silk fibroin woven mesh provides biocompatibility, breathability, elasticity, water-resistance, and strength - all in one dressing.
Made Without Cyanoacrylates
Engineered with a medical-grade pressure-sensitive adhesive designed to provide a gentle yet secure approximation, without any cyanoacrylate adhesives.
Zero Drying Time
Our pressure sensitive adhesive does not require drying time.
Water-Resistant
Water-resistant for routine showering when used as directed; avoid soaking or prolonged immersion.
Offloads Tension
Facilitates tension distribution across the incision when paired with subcuticular sutures.
Wear Up To 2 Weeks
Designed to stay in place for up to two weeks.
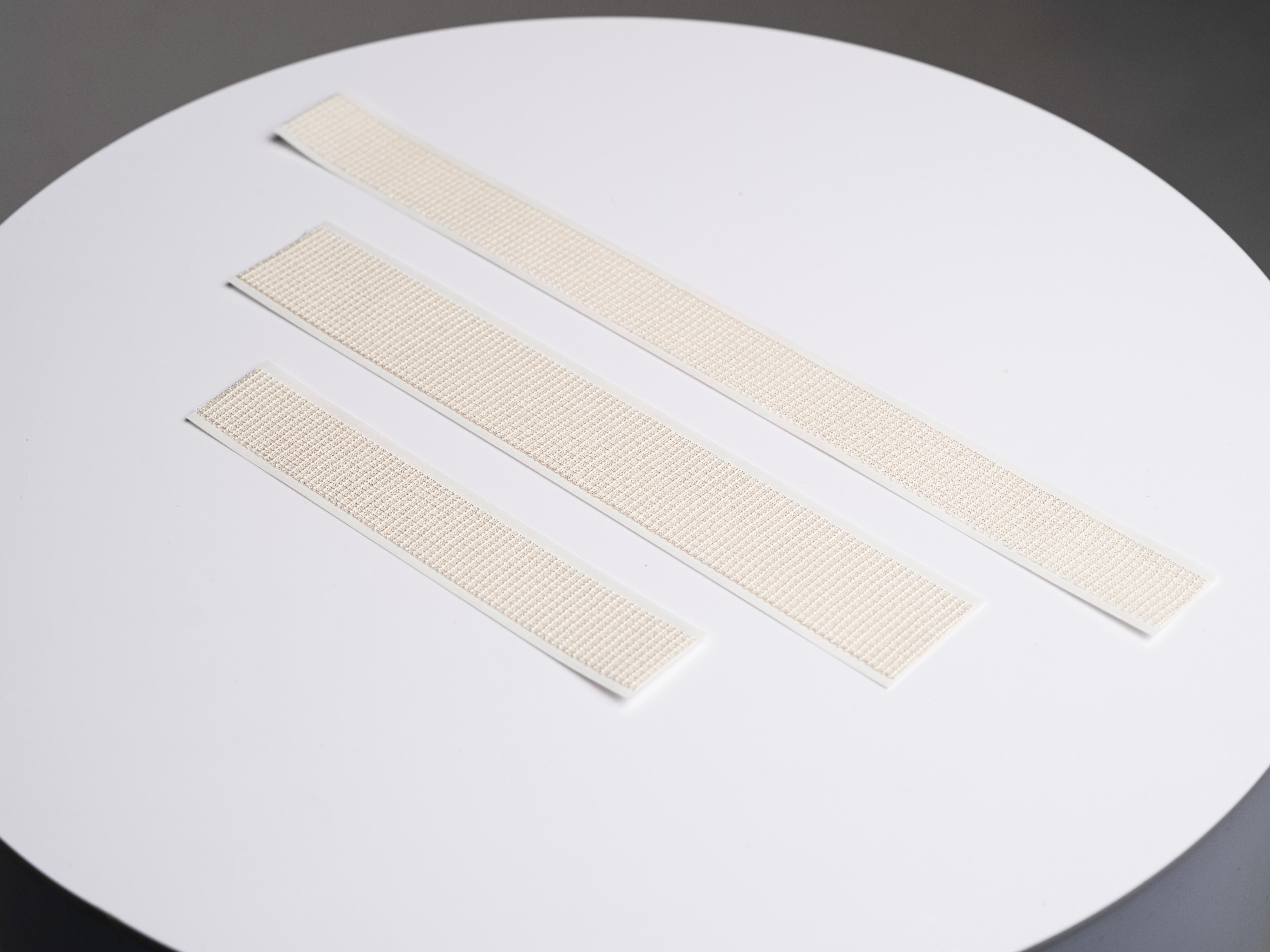
32cm, 24cm, or 16cm
3 Sizes Now Available


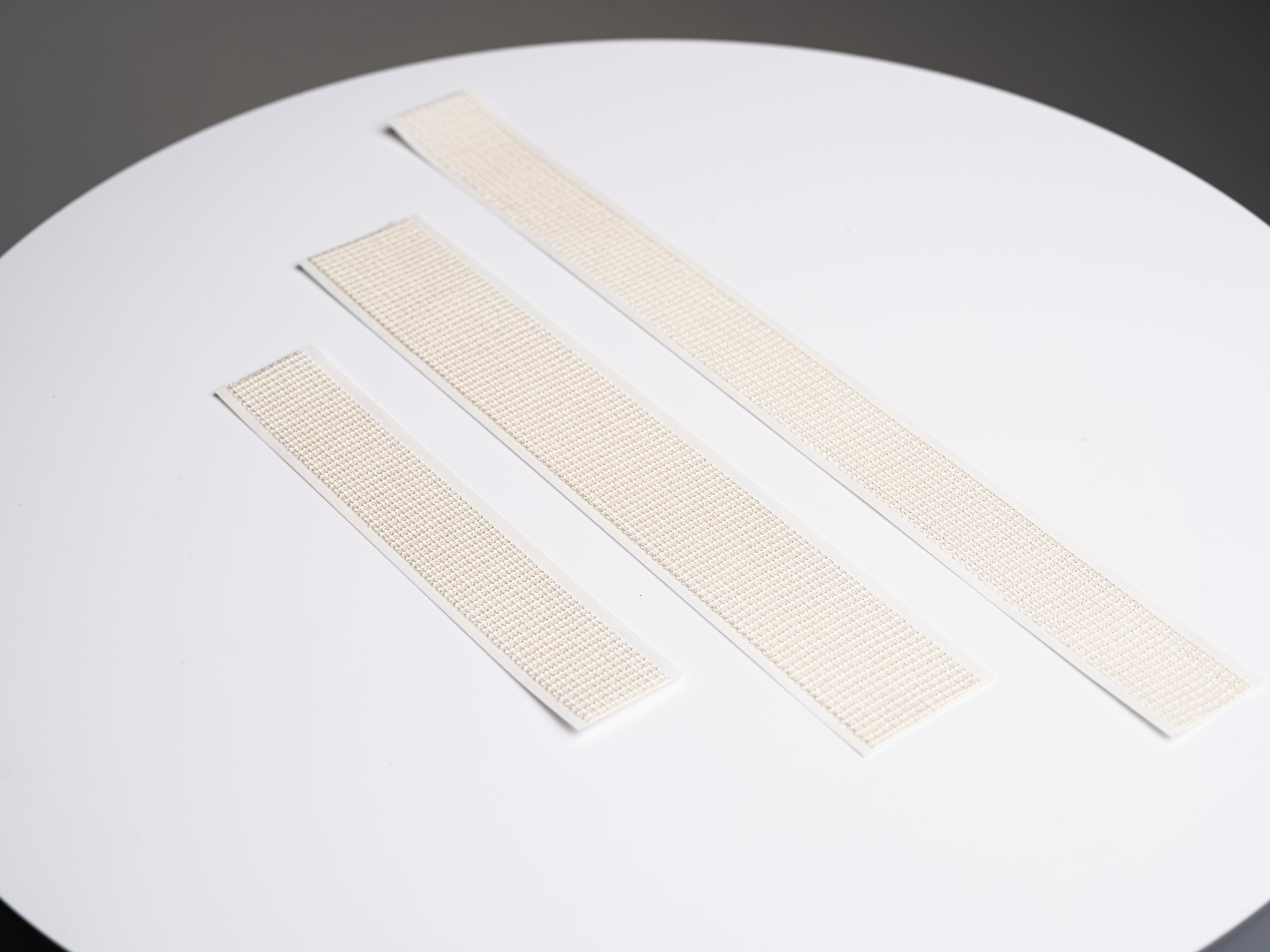






SYLKE® Adhesive Wound Closure
SYL-WC-32-010
AAHKS Industry Innovation Award (2025)
Breakthrough Designation with Premier GPO (2025)
Allure Best of Beauty Awards: Breakthroughs (2024)
Ships within 1 business day.
2-Year Shelf Life
Free FedEx 2Day® Shipping
Request Free Sterile Samples
What the clinical evidence is saying.
Follow us on social media and our newsletter to receive info on the latest clinical data.
Latest Stories
SYLKE® launches Global Relief: Charitable Use Program
Our charitable use program that partners with nonprofits and frontline healthcare teams to deliver SYLKE® Adhesive Wound Closure dressings where they’re needed most.
SYLKE® is now ISO 13485:2016–certified and MDSAP-audited
SYLKE® Adhesive Wound Closure is now ISO 13485:2016–certified and MDSAP-audited - supporting market access, pending regulatory approval, in the United States, Canada, Australia, Japan, and Brazil.