

The Founder's Story
SYLKE® was developed by M. Mark Mofid M.D., FACS, a practicing reconstructive and plastic surgeon with more than 20 years of experience. Dr. Mofid sought to create a product that better addressed the needs of surgical incision care.
During the early months of the COVID pandemic, with a lighter surgical schedule, he focused on addressing the challenges of surgical site care by designing a wound closure device using silk fibroin, a material known for its strength and biocompatibility.
Dr. Mofid is a clinical assistant professor of plastic surgery at The Johns Hopkins University School of Medicine and a highly regarded plastic surgeon with over 20 years of experience. Dr. Mofid graduated magna cum laude from Harvard University and earned his medical degree and completed his training at The Johns Hopkins School of Medicine.
He is a diplomate of the American Board of Plastic Surgery and the American Board of Facial Plastic and Reconstructive Surgery, as well as a fellow of the American College of Surgeons. Dr. Mofid also served as an Associate Professor of Plastic Surgery at the University of California, San Diego, for over a decade. He has presented his research at more than 40 national and international conferences and is the author of one of the most widely cited publications in plastic surgery.
"I envisioned a comfortable and effective skin closure device that meets the needs of both patients and practitioners."
— M. Mark Mofid, MD, FACSPresident and CEO
Timeline

2020
The Idea
During the early months of the COVID-19 lockdown, plastic surgeon M. Mark Mofid, MD FACS uses the pause in elective surgery to design a new wound-closure device made from silk fibroin.
2021
Prototype Created
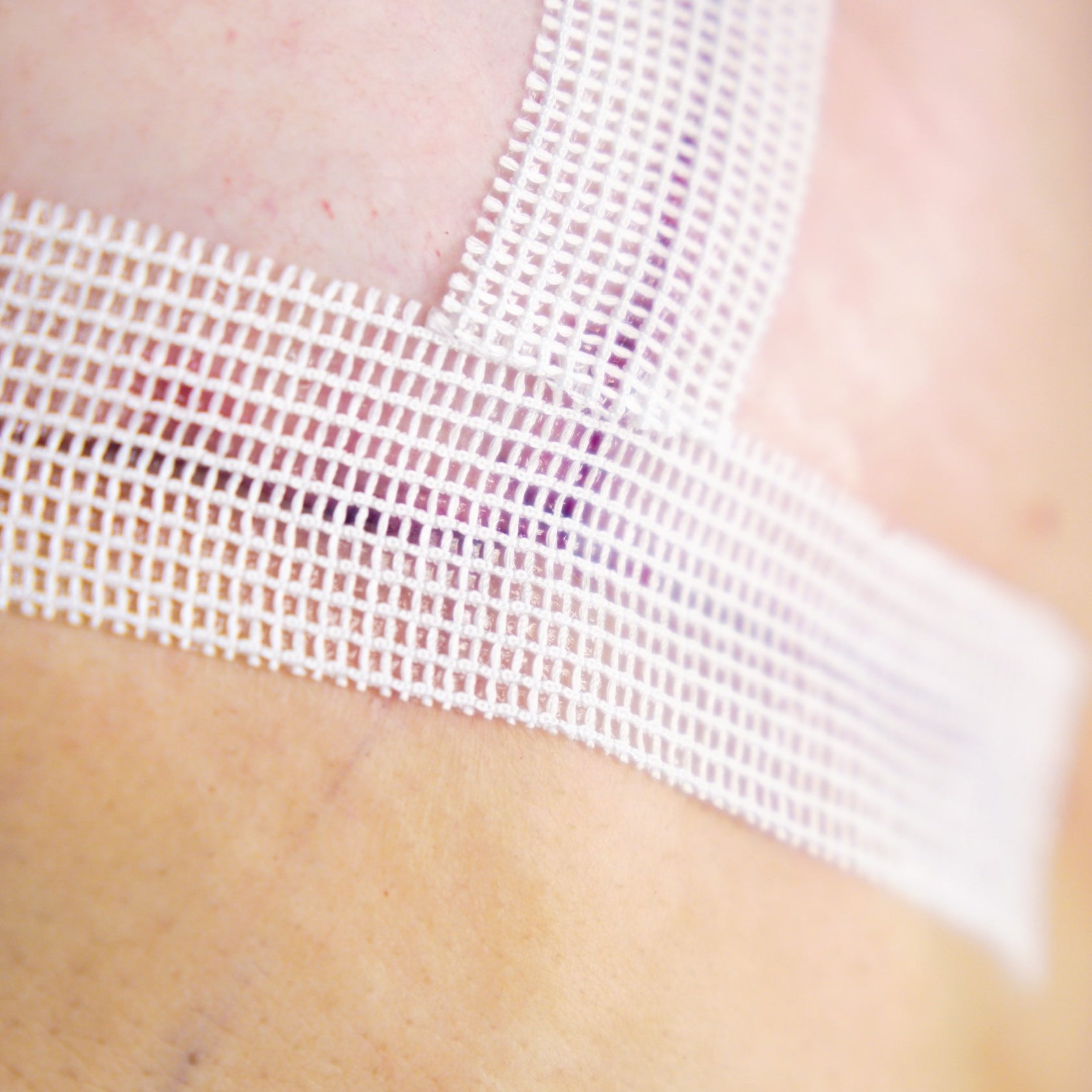
The following year the first prototype was created and the teams begins clinical trial preparation.
2022
Patents Issued & First RCT
Key patents are issued, establishing the intellectual foundation for SYLKE®’s silk bioprotein technology.
A single-blinded, prospective superiority trial enrolls 25 abdominoplasty and breast-reduction patients from May 11 to September 30, 2022, comparing a silk bioprotein dressing to Dermabond™ Prineo™ on opposite sides of the same incision—generating the first head-to-head clinical data.
2023
Commercial Launch
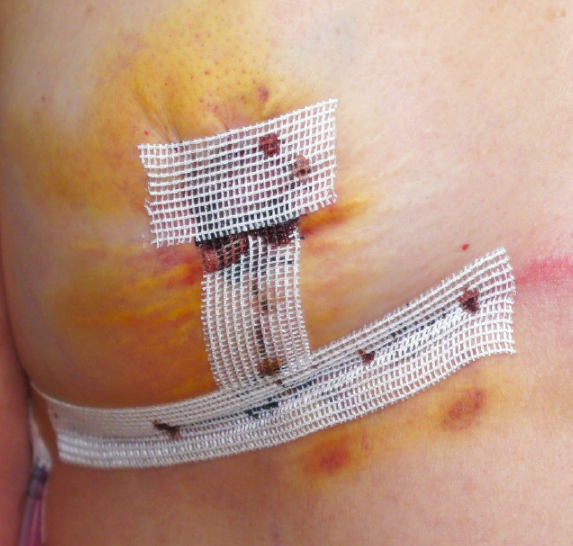
In December 2023, SYLKE® announces the commercial launch of SYLKE® Adhesive Wound Closure.
2024
Larger RCT & Seed Round
A second prospective, randomized, single-blinded clinical trial (50 patients) comparing a woven silk-fibroin dressing to 3M Steri-Strips™ is published in Plastic & Reconstructive Surgery.
Allure names SYLKE® Adhesive Wound Closure a 2024 “Best of Beauty Breakthrough.”

2025
Expansion & Series A
2025 was a breakout year for SYLKE®—three new peer-reviewed studies, two new sizes, ISO 13485 and MDSAP certifications, and entry into international markets.
This momentum was recognized with two prestigious honors: the AAHKS Industry Innovation Award and Breakthrough Designation with Premier GPO.
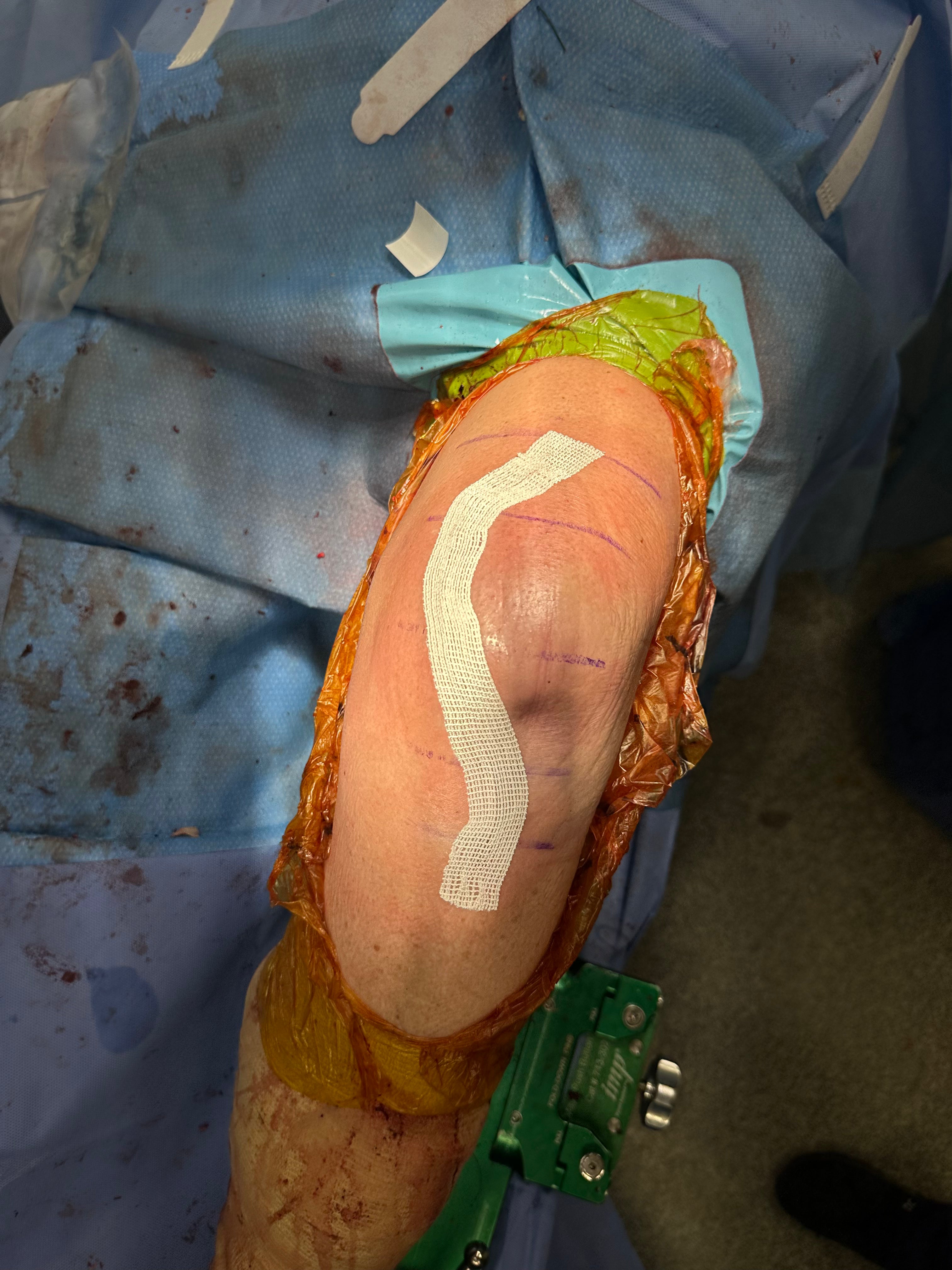
2026
Total Market Disruption
SYLKE® is on track to establish itself as the new standard of care, with accelerating clinical adoption driving expanded market presence in 2026.
Co-Founder

Sheva Mokarram, JD
Co-Founder, President of Global Affairs, Executive Vice President, Chief Legal Officer
Co-Founder, President of Global Affairs, Executive Vice President, Chief Legal Officer
Sheva Mokarram graduated from Texas Southern University, School of Law Summa Cum Laude in 2005 and is licensed by the California State Bar. She started her legal career as a civil litigation attorney, working at some of the most prominent national law firms in Los Angeles: Krohn & Moss Ltd, Selman Breitman LLP, Michelman & Robinson LLP, and Yee and Associates.
Her love of storytelling coupled with her legal background eventually helped her transition into the entertainment industry as a Television Executive at Reveille Productions. When the company was acquired by Murdoch's Shine Group, she departed with the managing partner to join a startup production company, INE Entertainment. For over a decade, she directed and produced thousands of hours of content across multimedia platforms, including television series, digital series, social media, and commercials. Sheva is a two-time Emmy-nominated producer for the Outstanding Culinary Program "Recipe Rehab" and Outstanding Children's Series "Everyday Health," and a consecutive winner of The Telly Awards for Outstanding Educational Culinary Program.
In March 2020, a moment of pandemic-era reflection sparked an unexpected new chapter. During a quiet morning conversation with her partner Mark, she asked what he would change about the field of surgery. That question—followed by five years of hard work, creativity, and collaboration—led to the founding of Sylke, Inc., now a nationally recognized leader in surgical skin closure and a new standard of care in medicine. As Sheva describes it, she and Mark are "the proverbial Yin and Yang, complementing one another with our strengths to create a whole greater than the parts."
Executive Team

Alexander Graham, JD
Chief Financial Officer, General Counsel
Chief Financial Officer, General Counsel
Alex joins SYLKE® as CFO and General Counsel, bringing more than a decade of combined legal and financial experience to his role at SYLKE®. Alex joins SYLKE® from Pillsbury Winthrop Shaw Pittman LLP, where he advised life-sciences companies and startups at all stages of growth. His practice focused on corporate governance, venture capital financings, mergers and acquisitions, and helping clients navigate the legal and strategic challenges of scaling their businesses. Prior to practicing law, Alex worked at KPMG where he specialized in economic and valuation services, advising multinational corporations on transfer pricing and business valuations. He earned a JD from the University of Michigan Law School and a BA in Finance from Michigan State University.

Scott Flowers
Executive Vice President, Vice President of Global Sales
Executive Vice President, Vice President of Global Sales
Scott Flowers joins SYLKE® as founding VP of Sales, bringing over a decade of expertise spanning sales, marketing, leadership, and operations. Most recently, Scott held the position of Southern California Sales Manager at Corza Medical, a pioneering startup specializing in wound closure, instruments, and hemostatic products. Prior to that role, he contributed to ConvaTec's success by selling wound, ostomy, skin, and critical care devices. In addition, Scott possesses experience selling biologic amniotic tissue and fluids. Throughout his career, Scott consistently demonstrated his prowess as a sales leader. He achieved numerous firsts, being the initial sales professional to introduce multiple new 510(k) technologies, pioneered innovative upstream marketing strategies targeting prominent players in the healthcare industry, and authored the playbook for countless medical professionals, guiding them through best practices for launching new medical products and services, spanning from Acute to non-Acute markets. Scott’s medical journey has cultivated robust relationships with hundreds of surgeons, clinicians, and executives across various specialties and health networks. His mission, similar to SYLKE®'s, centers on transforming challenges into opportunities, collectively reshaping the future of healthcare. He received his Bachelors of Science degree in kinesiology from California State University Northridge.

Becca Badowski
Senior Vice President, Chief Regulatory & Compliance Officer, Director of Operations
Senior Vice President, Chief Regulatory & Compliance Officer, Director of Operations
Rebecca joins SYLKE® as founding CRO, CCO, and Director of Operations to oversee all quality systems, regulatory, and compliance affairs. She is an accomplished Clinical Trial Specialist having designed multiple investigational and experimental studies. She personally managed the delicate care of hundreds of patients through complex and strict research protocols as well as tracking and reporting thousands of individual data points. She has successfully presented research initiatives multiple times in front of IRB panels, doctors, and research scientists. She brings enthusiasm for the strict details of compliance and uncompromising ethical standards required by the medical device industry. She received her Bachelor of Science from Indiana University.

Jack Ferrier
Senior Vice President, Chief Marketing Officer, Chief Technology Officer
Senior Vice President, Chief Marketing Officer, Chief Technology Officer
Jack joins SYLKE® as the founding CMO and CTO, where he is building the operational infrastructure that enables a lean team to sell and scale—driving lead generation, automating sales operations, and streamlining workflows to enable representatives to focus on surgeon relationships and patient outcomes. He brings an uncommon blend of frontline OR sales experience and self-taught software engineering. As a medical device representative at Nevro, PolyNovo, and ConvaTec, he consistently exceeded quota by bringing a technical edge to sell smarter. When COVID-19 disrupted traditional sales models, he led ConvaTec's national response, establishing an Inside Sales Team and launching digital go-to-market programs that earned multiple company awards. At SYLKE®, he applies that approach at the enterprise level. As CMO, he leads positioning, surgeon education, KOL development, and demand generation. As CTO, he oversees the technical systems and sales operations that support commercial execution. The dual role reflects his belief that marketing and sales operations are one and the same. Jack holds a Bachelor's degree in Film Scoring from Berklee College of Music.

Daniel S. Rouhani
Chief Scientific Officer
Chief Scientific Officer
Daniel Rouhani joins SYLKE® as a founding CSO, entrepreneur, scientist, and 3D design engineer whose work has been referenced in Forbes magazine, over 50 scientific news articles, and on the front cover of 10 peer-reviewed scientific journals including the journals of Science, Nature, and Cell. He was one of the first scientists to characterize and develop a comprehensive 3D model of the SARS-CoV-2 Nucleocapsid protein which was published and recognized internationally. He has presented his research at national and international research conferences and has spoken on a variety of research discussion panels. Rouhani is also the founder of ExonScientific, a 3D design company that pushes the boundaries of how science can be visualized through advanced 3D innovation. He has collaborated with over 30 institutions including the NIH, Harvard, Yale, and Northwestern on topics ranging from protein visualization, SARS-CoV-2 glycosylation, and genetic engineering. Rouhani is also a graduate of the National Science Foundation Innovation Corps program, has served as a judge for investor pitch competitions, and has taught courses in research and scientific writing. He graduated summa cum laude from the University of Georgia with a Bachelor of Science in Genetics and completed a year-long clinical research fellowship at the Johns Hopkins School of Medicine in the Department of Plastic and Reconstructive Surgery. He is currently completing his Doctor of Medicine degree at the University of California, San Francisco School of Medicine.

Setara Moosa
Senior Vice President, Chief Human Resource Officer, Controller
Senior Vice President, Chief Human Resource Officer, Controller
Setara Moosa joins SYLKE® as founding CHRO and Assistant Controller as a seasoned professional with over 25 years of experience in the medical field, specializing in sales, business management, and human resources. She has a strong background in leadership, team development, and operational efficiency, making her a key contributor to organizational growth and success.With extensive experience in human resources, Setara has been instrumental in recruiting, onboarding, employee relations, and compliance. She is skilled in fostering a positive workplace culture, implementing policies, and ensuring that business operations align with industry regulations. Her management expertise extends to strategic planning, financial oversight, and process improvement, helping businesses optimize productivity and enhance profitability. Having studied Business Management at San Diego State University, she combines her knowledge with hands-on experience to drive efficiency and innovation. Setara’s dedication to excellence and her ability to lead teams with integrity and vision continue to make a significant impact in the healthcare industry.
Team Members

Allen Castro
Director of Quality and Innovation
Director of Quality and Innovation
Allen joins SYLKE® as Director of Quality and Innovation as an experienced leader in quality management and innovation with extensive experience across big pharma, small pharma, medical devices, and startup environments. He excels in quality assurance, regulatory compliance, and product development, consistently driving safety and innovation within the life sciences sector. In his previous role, Allen spearheaded the design and development of novel RNA therapeutics, effectively bridging the gap between cutting-edge science and practical application. He oversees all aspects of quality management and product development, collaborating closely with cross-functional teams to uphold high standards throughout design and manufacturing processes. Allen is dedicated to delivering safe, effective, and innovative products to market, ensuring both regulatory compliance and market success. Allen holds a Bachelor’s degree in Chemistry from Vanguard University and a Master’s degree in Biotechnology from Rush University.

Bradley Wolf, MPAS, PA-C
Director of Medical Affairs
Director of Medical Affairs
Bradley Wolf is a Physician Assistant with over 20 years of experience in orthopedics and other surgical arenas. He serves as the Director of Medical Affairs at SYLKE®, where he integrates deep clinical expertise with innovative solutions to advance surgical care. Drawing from decades of patient care and operating room experience, Bradley provides the frontline perspective that ensures SYLKE®’s technologies address real-world surgical challenges. Bradley earned his undergraduate degree from Heidelberg University and completed his medical training in Pittsburgh at Chatham University. He currently works alongside Dr. Adolph Lombardi at JIS Orthopedics, specializing in adult hip and knee reconstructive surgery. Outside of medicine, Bradley enjoys an active life with his wife, Katie, a Nurse Practitioner, and their two children. A former collegiate soccer player, he continues to pursue his passion for sports and the outdoors as an avid golfer and skier.

Kathy Chea
Director of International Marketing and Expansion
Director of International Marketing and Expansion
Kathy Chea joins SYLKE® as Director of International Marketing and Expansion, bringing over 15 years of experience spanning global product management, marketing, and cross-functional leadership in the medical device and life sciences industries. Most recently, Kathy served as Director of Marketing and Regional Expansion at Corza Medical, where she led global lifecycle strategies and cross-functional initiatives to accelerate product development and market entry across surgical specialties including but not limited to plastic and orthopedic surgery. Prior to that, she held senior global marketing roles at Corza and Harvard Bioscience, launching products in international markets, negotiating distribution agreements, and driving adoption through targeted sales enablement and training programs. Throughout her career, Kathy has consistently bridged the gap between science and strategy—translating complex technologies into market-ready solutions that resonate with clinicians and drive business growth. She has cultivated strong relationships, empowered sales and distributor teams through tailored training, and led initiatives that expanded product portfolios and improved customer experience. Kathy holds an Executive MBA from Suffolk University and a Bachelor of Science in Biology with a minor in Chemistry from Westfield State College.

Christopher Rispoli
Global Director of Supply Chain
Global Director of Supply Chain
Chris Rispoli joins SYLKE® as the Global Supply Chain Director, bringing more than two decades of leadership in FDA-regulated, ISO-certified medical device and pharmaceutical operations. A seasoned supply chain and distribution executive, Chris is recognized for driving operational excellence, leveraging Lean methodologies, and delivering measurable improvements in cost, quality, and service across global networks. Most recently, Chris served as Director of Distribution at Corza Medical (formerly Angiotech/Surgical Specialties), where he oversaw all distribution center functions supporting domestic and international customers. He led cross-functional teams spanning receiving, inventory control, logistics, quality, import/export, and fulfillment. With full P&L ownership, Chris directed budgeting, regulatory audits, contract negotiations, and continuous improvement programs aligned with FDA, GMP, QMS, ISO 9001, and ISO 13485 requirements. Prior to Corza, Chris was Supply Chain Manager at Bose Corporation, managing multiple U.S. 3PL operations, implementing real-time reporting processes, and training teams in Lean problem-solving and risk-reduction methodologies. Earlier in his career, he supported clean-room operations and injectable pharmaceutical production for Baxter/VWR Scientific, ensuring 24/7 material availability in highly regulated, GMP-controlled environments. A Six Sigma/Toyota Lean–certified professional, he has extensive training in supply chain systems, continuous improvement, and regulatory compliance. His technical expertise includes deep experience with ERP platforms such as SAP and D365. Known for his hands-on leadership and collaborative style, Chris excels at building resilient teams, optimizing complex operations, and delivering scalable supply chain solutions for growth-oriented organizations. Chris studied Business at Northern Arizona University and is a veteran of the U.S. Navy.

Senko Ramadurai
Global Regulatory Affairs Manager
Global Regulatory Affairs Manager
Senko Ramadurai joins SYLKE® as Global Regulatory Manager bringing over 14 years of experience spanning product development, process engineering, quality engineering, and global regulatory affairs. He has contributed to major medical device programs across Corza Medical, BD, Stryker, GSK, McNeil Healthcare, and Terumo. Senko has led global regulatory strategy and EU MDR initiatives while supporting international market access and compliance. As a RAC-certified professional and ASQ-certified Quality Engineer/Auditor, he brings deep expertise in the design of Regulatory pathways, design controls, risk management, and post-marketing regulatory governance. Senko holds a Bachelor's in Biotechnology from Anna University and a Master's in Biotechnology from the University of South Florida. He is known for his cross-functional leadership, he consistently drives regulatory readiness, streamlined submissions, and global market expansion.

Gilad Touboul, MAB
Product Development and Quality Engineer
Product Development and Quality Engineer
Gilad joins SYLKE® as a Product Development and Quality Engineer, bringing broad and versatile experience across the medical device landscape. His engineering background spans biochemical, mechanical, electrical, firmware, software, and design engineering, complemented by a strong understanding of user needs and business priorities. With extensive experience in clinical medicine, pharma, medical devices, and startup environments, Gilad excels at guiding products from early concept through development, validation, and regulatory approval. He is committed to advancing innovation while ensuring the highest standards of safety and quality. Prior to joining SYLKE®, Gilad led the development and innovation of new product lines for both disposable and reusable healthcare devices. He is passionate about applying a whole-system perspective—integrating clinical insight, engineering expertise, and human-factors principles—to deliver safe, effective, and preferred products that truly benefit users and patients. Gilad holds a Bachelor's degree in Biology from the University of Oregon and a Master's degree in Applied Bioenginetic from the University of Washington.

Kelsea Amos
Human Resource Manager, Executive Assistant
Human Resource Manager, Executive Assistant
Kelsea Amos joins SYLKE® as an Human Resource Manager and Executive Assistant, bringing over a decade of experience in the plastic and reconstructive surgery field. With a wealth of knowledge and deep understanding of patient care, she holds a proven track record of outstanding administrative support. In her current role at Sylke as the Executive Assistant and Human Resource Manager, Kelsea is responsible for supporting executive leadership, managing human resource functions and ensuring seamless day-to-day operations. With her innate skills and experience she proficiently navigates both the healthcare and corporate landscapes, making Kelsea an instrumental and vital part of the Sylke team. Over the past 10+ years, Kelsea has built a diverse and successful career in the field of plastic surgery. Holding roles such as Certified Medical Assistant, Surgery Scheduler, Insurance Coordinator, and Assistant Manager. Her expertise in coordinating patient care and building key relationships with medical teams has been an invaluable asset to her current position. Kelsea’s approach in her role at Sylke brings a unique perspective and work ethic, one that blends operational proficiency with a genuine commitment in providing the best in customer care. Creating a positive workplace culture and supporting her colleagues reflects Kelsea’s dedication to the well-being of the team and the success and mission of Sylke.

Bryan Orosz
Sales Operations Manager
Sales Operations Manager
Bryan joins SYLKE®, now serving as Sales Operations Manager, overseeing and optimizing day-to-day sales operations to ensure the efficiency and success of the sales team. With 24 years of experience in sales support and customer service, Bryan brings a wealth of knowledge and expertise, particularly within the medical device sector. Bryan’s career began in technical support, where he honed his skills in industries such as telecommunications, calibration, and digital video security. He later transitioned into the medical device field, spending 13 years specializing in supporting reconstructive surgery and soft tissue repair device sales. During his time at ACell, Inc., Bryan played a key role in supporting the sales team and contributed to the design and continuous improvement of the company’s CRM system. Most recently, Bryan managed the Customer Relations department at Kerecis, collaborating closely with the sales team on Value Analysis and Credentialing to ensure smooth and effective communication between sales and healthcare providers. Driven by a passion for patient care, Bryan remains committed to improving outcomes through strategic operations and support. He holds an Associate Degree from Pennsylvania College of Technology.

Luke Wolfram
AI Automation and Data Specialist
AI Automation and Data Specialist
Luke joins SYLKE® as an AI Engineer, as a self-taught developer passionate about using software to solve real-world business problems. After releasing personal iOS apps, he worked with several startups—most notably Thrive Communities—where his client-database overhaul helped scale operations from 9 to 42 locations in 18 months. Focused on applied AI and systems design, he builds solutions that help reclaim time, reduce costs, and increase revenue. Luke studies Computer Science at the University of North Carolina.

Bhavay Chopra
Global Director of Systems and Automation
Global Director of Systems and Automation
Bhavay joins SYLKE® as an AI Engineer with a Master of Science in Data Science from the University of Colorado Boulder, where he specialized in predictive modeling, computer vision, and generative AI. He has applied his expertise across industries including manufacturing, pharmaceuticals, and digital accessibility—building solutions such as AI Agents for digital accessibility, malware prevention models, and sensor-based cold chain monitoring for supply chains. Professionally, Bhavay has contributed to data science and analytics roles at companies like Hill & Smith Inc. and Innominds Software, where his work improved decision-making through advanced dashboards and AI-powered monitoring tools. Passionate about creating scalable AI products, he is focused on driving innovation and real-world impact at SYLKE®.

Brooke Dunford
Marketing Specialist
Marketing Specialist
Brooke joins SYLKE® as a Marketing Specialist, bringing a fresh and innovative perspective to her role and the company. With seven years of customer service experience, she maintains a customer-centric approach and values creating memorable experiences across every touchpoint. At SYLKE®, Brooke turns insights into unified, omnichannel campaigns and utilizes consumer feedback and KPIs to guide strategy and measure impact. Curious and motivated, she’s committed to continuous learning and to contributing meaningfully to the healthcare industry. Brooke earned a B.S. in Marketing and Entrepreneurial Management from the University of Minnesota Twin Cities.

Crystal Reign
Manager of Global Education and Conferences
Manager of Global Education and Conferences
Crystal Reign joins SYLKE® as Executive Assistant, bringing a foundation in legal administration, finance, and biomedical sciences. She began her career in public healthcare administration with the Oklahoma Healthcare Authority, supporting contract development and provider relations. Joining SYLKE® in its formative stage, she led initiatives to establish core resources and go-to-market foundations that enabled early engagement and growth. Today she oversees conferences and events, supports executives and the sales team, and flexes across operations—driven by the intersection of healthcare, technology, and business innovation. Crystal holds an Associate’s Degree in Legal Administration from OSU-IT in Okmulgee, Oklahoma; a Bachelor’s Degree in Finance from Northeastern State University in Tahlequah, Oklahoma; and a Graduate Certificate in Medical Sciences from OSU College of Osteopathic Medicine in Tulsa, Oklahoma.

Jennifer Hsu
International Market Strategy Coordinator
International Market Strategy Coordinator
Jennifer Hsu joins SYLKE® as International Market Strategy Coordinator, focusing on distributor mapping, regulatory planning, and partner outreach across various territories. She brings data-driven experience from working on Eli Lilly clinical trials and academic research, combining statistical analysis and market intelligence with execution. At SYLKE®, Jennifer builds distributor datasets, sizes markets, conducts competitive and customer market research, and supports regulatory pathways. Beyond SYLKE®, she has presented research at national conferences. Jennifer attended Brown University in Applied Math–Economics and Health & Human Biology and plans to complete her MD at Warren Alpert Medical School.

Ashley D'Ambra
Customer Relations and Business Development Team Lead
Customer Relations and Business Development Team Lead
Ashley joins SYLKE® to help accelerate commercial growth, bringing a strong track record in healthcare solutions and market-intelligence sales. She launched her sales career at BCC Research, advising senior decision-makers with market-research insights, then built a new-business pipeline at Aberdeen by selling content marketing, data, and lead-generation programs across diverse industries. For the past six years, she has served as a Client Solutions Executive at Corza Medical, expanding new accounts and strengthening major hospital-system relationships for wound-closure products—experience she will apply to uncover new market opportunities, build scalable sales processes, and drive sustained revenue growth.
Advisory Board Members

Robert S. Gorab, MD
Advisory Board Member
Advisory Board Member
Robert S. Gorab, MD is the Tom & Mayumi Adams Endowed Chair in Orthopedic Surgery and the Immediate Past Chief Medical Officer of the Hoag Orthopedic Institute (HOI) in Irvine, CA. He is the Program Chairman of the Adult Reconstruction & Joint Replacement Fellowship Program at that institution. As a founding member of HOI, Dr. Gorab is actively involved in research, surgeon education, and the design and development of orthopedic implants and procedures. Dr Gorab received his undergraduate education at Colgate University. After graduating from medical school at the University of Pittsburgh, Dr. Gorab completed his residency in orthopedic surgery at UCI Medical Center, followed by fellowship training in adult reconstructive surgery at the Cleveland Clinic. He has served as an associate professor of orthopedics at the University of California, Irvine School of Medicine and is a member of the American Association of Hip and Knee Surgeons, the American Orthopedic Association, the American Academy of Orthopedic Surgeons, the California Orthopaedic Association and the Western Orthopaedic Association. He and his wife, Lisa, a pediatrician, have two adult children. A former collegiate soccer player, Dr. Gorab continues to enjoy sports and the outdoors. He is an avid cyclist and downhill skier.

Steven Barnett, MD
Advisory Board Member
Advisory Board Member
Steven Barnett, MD is the Chief Medical Officer as well as a board member of the Hoag Orthopedic Institute in Irvine, California and a managing partner of the Orthopedic Specialty Institute in Orange County. He is a board-certified orthopedic surgeon with fellowship training specializing in Adult Reconstructive Surgery. His practice is limited to joint replacement and arthritis surgery of the hip and knee, and he performs over 600 joint replacement surgeries per year. He received his undergraduate degree at the University of California, Berkeley. Subsequent to this, he attended Boston University School of Medicine for his medical degree. He then went on to complete his orthopedic residency at University of California, Irvine. Following residency, he completed an additional fellowship year in joint replacement and adult reconstructive surgery at the Institute for Bone and Joint Disorders in Phoenix, Arizona. He is currently an active participant in both implant and instrument design as well as research and physician education through the Hoag Orthopedic Adult Reconstruction Fellowship program. Dr. Barnett holds membership in the American Association of Hip and Knee Surgeons as well as the American Academy of Orthopedic Surgeons.

William J. Long, MD, FRCSC
Advisory Board Member
Advisory Board Member
Dr. William J. Long is a board-certified Attending Orthopedic Surgeon at HSS specializing in adult hip and knee reconstruction, revision joint replacements and sports injuries of the knee. He is a member of a number of prestigious societies including the Knee Society, where he is on the Research Committee, and the Hip Society. He is a board member, and Knee Society representative on the American Joint Replacement Registry Steering Committee. He serves on a number of internal committees at HSS, and is the Chairman of the Business and Strategic Growth Committee in the ARJR Division. He is an expert in advanced technology, and has helped to design and introduce two robotic platforms to market, both of which aid surgeons in better performing total knee replacements. Dr. Long previously served as an attending orthopedic surgeon at the Insall Scott Kelly Institute for Orthopaedics and Sports Medicine in New York City. He was also a clinical associate professor at NYU Langone Orthopaedic Hospital and assistant program director of the ISK/NYU Adult Reconstruction Fellowship, as well as former director of research at St. Francis Hospital in Roslyn, New York. He directed and ran the ISK Annual meeting in New York for 10 years. He received several recognitions, including the Dr. Jaffe Fellow Research Award 5 consecutive years at NYU, and received the teaching award. Dr. Long completed his undergraduate degree at Queen's University at Kingston, in Ontario, Canada, and his medical degree at the University of Western Ontario. He completed a residency in orthopedic surgery, also at Queen's University, a fellowship in lower extremity adult reconstruction at the Mayo Clinic and a second fellowship in sports medicine and reconstruction at the Insall Scott Kelly Institute. More recently he completed the Harvard Surgical Leadership Program and won the Clinical Change Team Award. The author of more than 100 peer-reviewed papers in the last decade, Dr. Long is also editor and author of a number of textbooks on hip and knee reconstruction. He is an invited speaker at numerous national and international meetings on an annual basis. Dr. Long is the recipient of the 2019 Frank Stinchfield Award from the Hip Society, as well as the first-ever Tri-Org grant from the American Academy of Hip and Knee Surgeons and the Hip Society and Knee Society.

Mark Figgie, MD, MBA
Advisory Board Member
Advisory Board Member
Mark Figgie, MD is a Professor of Surgery and Chief Emeritus of the Surgical Arthritis Service at the Hospital for Special Surgery. He received his Chemical Engineering and Economics degrees from Bucknell and his medical degree from Case Western Reserve University. He received his MBA from the NYU Stern School of Business. He is the Chairman of the Board of Wishbone Medical, Inc.

Vasili Karas, MD, MS
Advisory Board Member
Advisory Board Member
Dr. Vasili Karas is a board-certified, fellowship-trained orthopedic surgeon specializing in rapid recovery hip and knee replacement and complex reconstruction surgery, practicing in Chicago, Illinois. He is also an Assistant Professor at Rush University Medical Center. A Chicagoland native, Dr. Karas earned his medical degree from Rush University Medical College in Chicago. He then completed his residency training in orthopedic surgery at Duke University Medical Center in North Carolina. Following his residency, he returned to Chicago to complete a fellowship in hip and knee replacement surgery at Rush University Medical Center. Dedicated to advancing his field, Dr. Karas is an active committee member of the American Academy of Hip and Knee Surgeons (AAHKS). He has also authored over 50 peer-reviewed articles. He is privileged to provide care to both his local community and individuals from around the world, aiming to restore mobility and enhance the quality of life for all his patients. He practices at several locations affiliated with Rush, including Rush University Medical Center, Rush Oak Park Hospital, and The Lincoln Park Center for Advanced Orthopaedic Surgery.

Li Poa, MD, FACS
Advisory Board Member
Advisory Board Member
Li Poa, MD is a Professor of Cardiovascular Surgery at Columbia University College of Physicians and Surgeons and a former Associate Professor at the UCLA David Geffen School of Medicine. He received his undergraduate and medical degrees at Northwestern University where he also holds a Masters in Management from the Kellogg Business School. Dr. Poa completed his cardiothoracic surgical fellowship at UCLA and is board certified. He is most well known for his pioneering work in the fields of beating heart minimally invasive coronary bypass surgery, endoscopic cardiac surgery, mitral valvular repair, and thoracoscopic ablation surgery. He currently serves as the Chief of Cardiovascular and Thoracic Surgery at the Garfield Medical Center and Centinela Hospital in Los Angeles.

Curt J. Welker, CPA
Advisory Board Member
Advisory Board Member
Mr. Welker began his public accounting career in 1982, after serving as an assistant controller and later Chief Financial Officer for international trading firms, as well as spending four and a half years with a Big Four firm. His tax specialties encompass real estate, hospitality, manufacturing, individual taxation—including estate tax planning—and corporate tax planning. An active member of several professional organizations, Mr. Welker is frequently sought after for his insights and has been quoted on accounting and tax matters in both local and national publications. Mr. Welker received his Bachelor of Science degree from San Diego State University, subsequently undertaking graduate coursework in taxation.
Advisors

Dino Montagner
Advisor
Advisor
Dino Montagner was an expert in the manufacturing and distribution of the finest silk textiles in the world, establishing a global business across Italy, China, and India. Over 40 years ago, Mr. Montagner fell in love with silk; however, he decided to pursue a different direction than his high-fashion contemporaries. Mr. Montagner devoted his life and career to the discovery and use of silk fibroin as a biomaterial. From this vision, he developed a full range of therapeutic undergarments to aid dermatology patients and forged new pathways for gentle skin regeneration of wounds with silk and antimicrobial combinations. Backed by over 20 Clinical Studies and global patents for multiple medical devices and applications, Mr. Montagner was as professional as he was passionate about the healing properties of silk.

Eva Montagner
Advisor
Advisor
For nearly 30 years, Ms. Montagner has worked in the family business, which she co-founded with her father in 1997, with the mission of improving the quality of life of patients with allergic and dermatological conditions through innovative medical textiles. She has participated in the development and patenting of innovative medical devices, including a complete line of therapeutic undergarments made from microbiologically protected silk fibroin for the prevention and treatment of skin and mucosal conditions. This MD is supported by over 20 published clinical studies and is recognized by the national health system in several European countries. In recent years, she has joined her father in the research and development of silk fibroin as a biomaterial developing a patent. She also gained experience in sales, marketing, communications and regulatory affairs within the company. She took over the management of the company from her father in 2024.

Navin Singh, MD, MBA, MS
Advisor
Advisor
Dr. Navin Singh is an award-winning dual-board-certified plastic surgeon. He has received prestigious credentials from Brown and Harvard and formerly served as a director for cosmetic surgery at Johns Hopkins University School of Medicine. Being an expert in his field, Dr. Singh is also a board examiner for both the American Board of Plastic Surgery and the American Board of Facial Plastic and Reconstructive Surgery. As one of the top plastic surgeons in Northern Virginia, his expertise is evident through his multifaceted involvement in the field as he continues to author numerous medical publications and lecture medical professionals nationally to discuss his surgical knowledge and insight. Dr. Singh is featured on numerous media outlets such as CBS News, ABC News, and The Doctors on NBC. He has been consistently voted as a Top Doc in Washingtonian, Bethesda, and Northern Virginia magazines to discuss his medical insight and surgical expertise.

Jason D. Toranto, MD
Advisor
Advisor
Dr. Jason Toranto is a dual board certified plastic surgeon with fellowship training in craniofacial and pediatric plastic surgery. He began practice at the University of California, Irvine where he was an Assistant Professor, Co-Director of Research, and Director of Craniofacial surgery. He then became the Chief of Plastic Surgery at a multi-disciplinary clinic, where he focused on complex facial reconstruction, and now is in private practice. His current practice focuses on the interplay between plastic surgery and other surgical subspecialties, providing tailored reconstructive and aesthetic options to his patients. Dr. Toranto has published numerous articles on a variety of subjects, including fat grafting, hypertrophic scarring, hernia repair, and lower extremity reconstruction. He is also the CEO and Co-Founder of Health Allies Clinic (HAC), a multi-disciplinary medical group that focuses on providing specialized surgical services to American College of Surgeons (ACS) Level One - Three Trauma Centers. Under Dr. Toranto’s leadership, HAC has successfully stabilized emergency healthcare delivery across a significant region of San Diego County. Dr. Toranto received his Bachelor of Science (B.S.) with honors from Stanford University and his Medical Doctorate (M.D.) from the University of Michigan.

Alberto J. Arguello, MD
Advisor
Advisor
Dr. Alberto J. Argüello is a Costa Rican board certified plastic and reconstructive surgeon. He graduated with honors from the University of Costa Rica and then from the University of Guadalajara, Mexico in March, 2005. He is the founder and CEO of the Advanced Center of Plastic Surgery in San José, Costa Rica. He has also served as the Assistant Chair of National Secretaries and belonged to the Patient Safety Committee of the International Society of Aesthetic Plastic Surgery. He is a three time President of the Costa Rican Society of Plastic, Reconstructive and Aesthetic Surgeons as well as a member of the Aesthetic Surgery Journal Editorial Board. For the past 14 years, Dr. Argüello has served on the Board of Directors of American Association for Accreditation of Ambulatory Surgery Facilities. He has been a visiting professor to many plastic surgery meetings worldwide and is regularly invited to appear on morning Television shows in Costa Rica’s Channels 6, 7, and 8.

Adah Almutairi, PhD
Advisor
Advisor
Dr. Almutairi is one of Forbes's top ten most influential female engineers in the world, a scholar of science and engineering, an inventor, and an entrepreneur. Dr. Almutairi is a professor of pharmaceutical chemistry; faculty in the departments of Bioengineering and NanoEngineering; and director of the Center for Excellence in Nanomedicine and Engineering at the Institute of Engineering in Medicine at the University of California, San Diego (UCSD). Her work focuses on nanomedicine, nanotechnology, chemistry, and polymer science. Almutairi is a 2016 Kavli Fellow and has received numerous honors and awards such as the NIH director's new innovator award in 2009 for her work on "Chemically Amplified Response Strategies for Medical Sciences". Almutairi's groundbreaking work was highlighted by U.S. NIH director Francis Collins to Congress as one of the 4 most important American technology breakthroughs of the year 2012. Almutairi holds 13 U.S. patents issued, some of which are currently licensed in the Industry. She is a consultant for Fortune 500 companies (IBM, BASF, Roche, Pfizer, Merck) in the Chemical and Pharmaceutical industry and is the Founder of eLux Medical Inc. She is a Technology Investment Advisor to Angel groups, family offices, and venture funds. Almutairi also holds 10 years of experience as a U.S. government Science policymaker (NIH, NSF, AAAS, U.S. Army) with a focus on Research & Development and Innovation for a Knowledge-based economy.Dr. Almutairi received her Bachelor of Science from Occidental College and her PhD from the University of California, Riverside.

James Chao, MD, FACS, PhD, MBA
Advisor
Advisor
James J. Chao, MD, FACS is a board-certified plastic surgeon and the CEO of Prime Plastic Surgery, Prime Med Spa, and Oasis MD Lifestyle Healthcare. Dr. Chao is a fellowship-trained hand and microvascular surgeon who specializes in cosmetic and reconstructive surgery of the hand and upper extremity, aesthetic and reconstructive breast surgery, body contouring surgery, and the development of new technologies in the field of plastic surgery. Four years ago, Dr. Chao left his tenured position as a Clinical Professor of Surgery at UCSD and started Peak Healthcare Alliance (PHA) in response to the changing healthcare landscape. Along with co-founders George Scopetta and Dr. David Chao, he currently has amalgamated multiple companies and established strategic partnerships into a vertically integrated healthcare delivery platform with assets in excess of $250M. PHA is specifically designed to leverage the economics of today’s healthcare environment into a sustainable premium consumer-driven brand of lifestyle medicine for the future.Dr. Chao received his Bachelor of Science from Stanford and his MD degree from NYU.

Thomas Mitchell, MBA
Advisor
Advisor
Thomas M. Mitchell is the managing partner of Crestmont Ventures, providing venture assistance and strategic advice to entrepreneurs working on breakthrough technologies that solve identifiable problems. At Crestmont, Thomas has invested in over 50 companies and created 5 billion dollars in value with 15 successful exits. He has over 50 years of experience consulting for global start-up ventures. Thomas is a U.S. Air Force veteran with a BA from Yale University and an MBA from the University of Denver.

Alain Schreiber, MD
Advisor
Advisor
Dr. Alain Schreiber served as Managing Partner of Proquest Investments, a healthcare venture firm with over $900M in assets under management from 2000 to 2021. In this capacity, he served on over 20 boards including 7 public companies and oversaw 17 exits by merger or public offerings. Prior to Proquest Dr. Schreiber was the CEO of Vical, a public vaccine biotechnology company, from 1992-2000. Previously he served as Senior VP of Research at Rhone Poulenc Rorer (now part of Sanofi) and Department Head of Biochemistry at Syntex (now Roche). Dr. Schreiber received a B.S. in chemistry, summa cum laude, and an M.D., summa cum laude, from the Free University in Brussels, Belgium. Subsequently, he was a postdoctoral fellow at the Weizmann Institute of Science in Israel.
Interested in joining?
Reach out to our team and if there's a fit, we'll be in touch.

